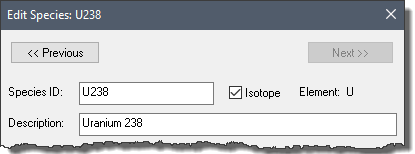
In some models, you may need to simulate species which are isotopes of the same element.
You specify that a species is an isotope by checking the Isotope check box to the right of the Species ID:

If the box is checked, the isotope's Element is displayed to the right of the Isotope check box. GoldSim defines the Element as the character(s) to the left of the first number in the species ID (e.g., the element name for Am241 would be Am). Therefore, it is recommended that isotope species be named as EEnnn, where "EE" is the element abbreviation, and "nnn" is the mass number (e.g., Am241, U238, C14).
 Note: This is not necessary if
you are using species that are included in the list from the ICRP Database
provided by GoldSim within the RT Module (since GoldSim will automatically mark
the appropriate species as isotopes (and identify their elements).
Note: This is not necessary if
you are using species that are included in the list from the ICRP Database
provided by GoldSim within the RT Module (since GoldSim will automatically mark
the appropriate species as isotopes (and identify their elements).
 Note: If none of the species
are defined as isotopes, the Species array label set
and the Elements array label set will have the same number of entries,
but may be in a different order. The Elements array label set is always in
alphabetical order, while the order of the Species array label set is determined
by the user.
Note: If none of the species
are defined as isotopes, the Species array label set
and the Elements array label set will have the same number of entries,
but may be in a different order. The Elements array label set is always in
alphabetical order, while the order of the Species array label set is determined
by the user.
Specifying multiple species as isotopes of the same element (e.g., U235, U238) has two effects. First, isotopes of the same element are assumed to be chemically equivalent (i.e., behave identically on a molar basis with regard to partitioning, solubility, and diffusivities). These media-specific properties are defined within the Fluid and Solid media elements.
Within a Fluid or Solid element, properties (such as solubilities and partition coefficients) can be defined by species or by element. If they are defined by species, GoldSim forces the properties of species that are isotopes of the same element to be identical. In particular, all isotopes of the same element take on the properties of the first of the element's isotopes in the species array label set. For example, if the species array label set included U234, U235, and U238 (in that order), all three species would use the properties (e.g., molar solubilities) assigned to U234, regardless of what was specified for the other two species.
The second effect of defining multiple isotopes of the same element is related to how solubilities are computed in Cell pathways (which are the only type of transport pathway where solubility constraints can be applied). In particular, when computing solubility constraints in Cell pathways, the solubility is "shared" by (split among) all isotopes of the same element.
Learn more about:
Creating and Editing Environmental Media